1. RAPID AUTOMATED RESULTS YOU CAN TRUST
- Minimal hands-on time for specimen preparation (1 min)
- Test results in as little as 30 minutes*
- Read on-screen results, optionally view curves
- Multiplex capability using microfluidics to perform rapid, six channel PCR testing in real-time
- Built-in quality control
2. EASY TO USE SYSTEM
- Tests are simple to perform, using easy to follow guided workflows
- Simple, intuitive, software available in local languages
- Large, responsive 7 inch wide-screen touch display
- No calibration required and mimimal user maintenance (air filter)
3. CLOSED AND SAFE SYSTEM
- Reduced contamination risk
- Fully sealed cassettes
- Reagents integrated into the test cassettes
- Integrated barcode scanner toidentify test cassettes and initiates the test run process
4. FLEXIBILITY AND CONNECTIVITY
- Small footprint ( W 24cm x D 30cm x H 20cm ) and weights less than 8kg
- Multiple connectivity options: LIS, LAN, 3G, 4G and WiFi
- Mains operated system with internal battery (five runs on a full charge)
- Seamless transition between mains and battery in the event of a power disruption

PRINCIPLE OF OPERATION
The Q-POC™ is compatible with single-use QuantuMDx test cassettes and possesses the ability to manipulate fluid and generate the conditions required to perform polymerase chain reaction (PCR) and report qualitative fluorescence signal data. The instrument is designed to be easy to use and require minimal instrument maintenance or operator training.
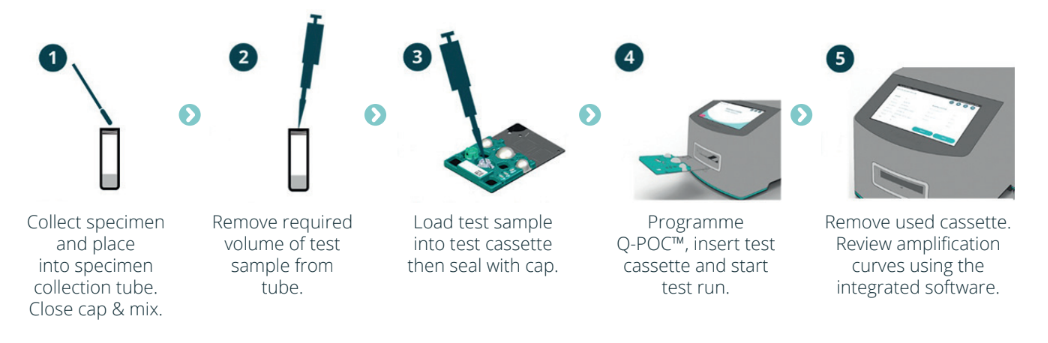
The general Q-POC™ testing workflow consists of five (5) main operator steps:
Q-POC is available in the following A.Menarini Diagnostics countries:
France: https://www.menarinidiagnostics.fr
UK: https://www.menarinidiag.co.uk